- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
Noémie Lalaoui, Marcello Gennari, Carole Duboc, Jérôme Chauvin, Fabrice Thomas, Olivier Jarjayes, Catherine Belle, Aurore Thibon-Pourret, Lili Sun (postdoc), Kabibi Kamashanju (PhD), Kaiji Shen (postdoc)
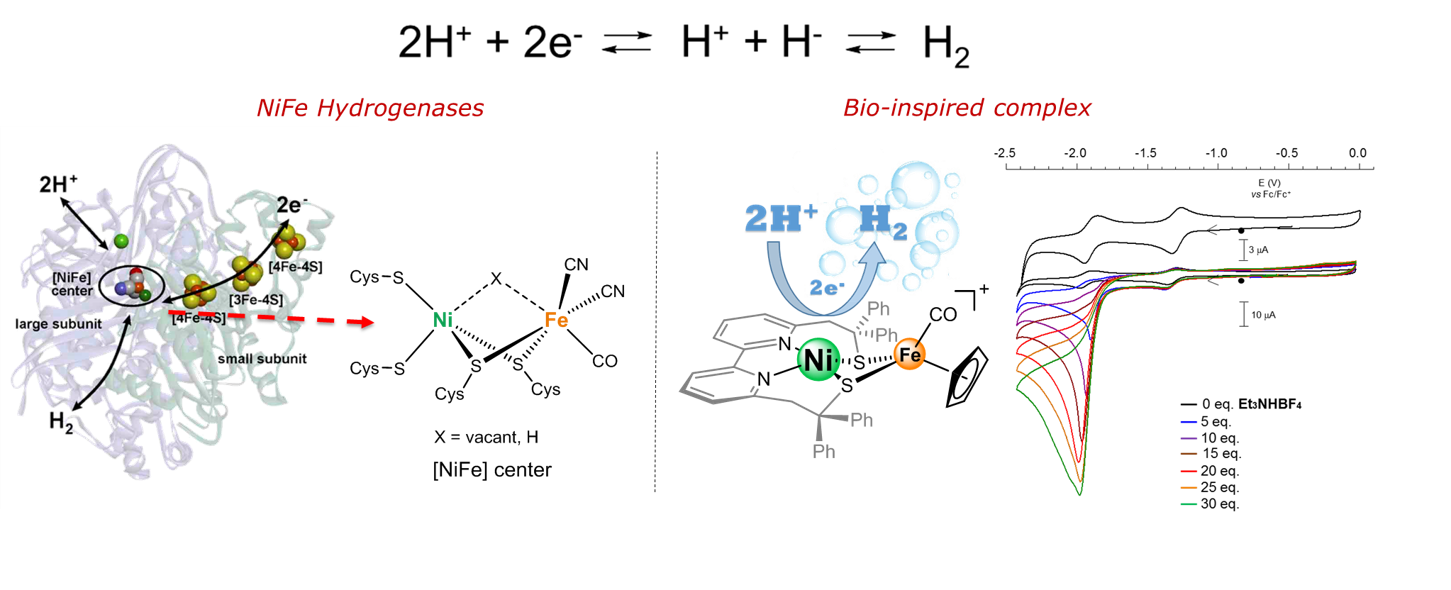
Dioxygen reduction is an essential reaction, which can lead to substrate oxidation and the generation of electricity in fuel cells for example. The number of electrons involved can vary from 1 to 4 and the control over this number is crucial for targeting any application. We develop in our team coordination compounds for chemical or electrochemical dioxygen reduction (homogeneous and heterogeneous). By an appropriate ligand design and a judiciously chosen metal ion we demonstrated that we can finely tune the reactivity (e.g. the number of electrons). Carbon nanotubes can be functionalized (collaboration with the BioCEN team) with some of these complexes leading to new generations of fuel cells. On the other hand, we target transition metal complexes that mimic the active site of [Ni·Fe] hydrogenase in order to develop efficient catalysts for H2 production. We have synthesized a structural and functional model whose redox reactivity is centered on the nickel center as in the enzyme. We have also detected an intermediate involved in the catalytic H2 production cycle that mimics a [NiFe]-hydrogenase intermediate (Ni-L state). The kinetics of hydrogen production catalyzed by the [NiFe]-mimic unambiguously demonstrates the synergy between nickel and iron in driving the process. A comparative evaluation of the performances of the nickel-iron catalyst reveals that these far surpass those of the other dinuclear biomimetic models.
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn