- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
Overview
The EMPRe team conducts research in the field of molecular electrochemistry and redox photochemistry toward the understanding of bond activation triggerred by electron transfer. Within this framework, we are developing and analyzing electrochemical and photoinduced redox catalytic processes in homogeneous solution and on surfaces. Students in the EMPRe team are trained in synthesis of ligands, transition metal complexes or organic dyes. Then, they apply methods of molecular electrochemistry (cyclic voltammetry, electrolysis), spectroelectrochemistry, surface modification (electropolymerization, electrodeposition) to study mechanisms of electro/photo-catalytic processes. In this context, we are investigating in particular small molecule activation (such as H2O, CO2, N2O) as well as proton-coupled electron transfer processes... To provide a rational approach to mechanistic study and an intelligent design of molecular catalysts, we are developing kinetic and theoretical models related to cyclic voltammetry and electro-photo-catalytic processes.
In this section

Permanent researchers
Sylvie Chardon (DR CNRS), Marie-Noëlle Collomb (DR CNRS), Cyrille Costentin (PR UGA), Gabriel Durin (CR CNRS), Jérôme Fortage (CR CNRS)
Awards
2025 Poster Prize of Symposium 12 on "Molecular spectro-photo-electrochemistry and electrosynthesis" at the 76th annual meeting of the International Society of Electrochemistry awarded to Céline Naddour
2025 Best Oral Presentation Prize at the "Journée de Printemps" of the Rhône-Alpes section of the Société Chimique de France awarded to Céline Naddour
2024 Master 2 internship Prize from the Electrochemistry Subdivision of the Société Chimique de France awarded to Margaux Willery
2024 Jaroslav Heyrovsky Prize for Molecular Electrochemistry from the International Society of Electrochemistry awarded to Cyrille Costentin ("for fundamental studies explaining a general framework of PCET catalysts operation in particular in relation to small molecules activation")
2023 Innovation Prize from the Division Chimie Physique of the Société Chimique de France awarded to Marie-Noëlle Collomb and her team
2023 Master 1 internship Prize from the Electrochemistry Subdivision of the Société Chimique de France awarded to Alexandra Collard
2022 Senior Researcher Prize from the Division Chimie Physique of the Société Chimique de France awarded to Cyrille Costentin
Research activities
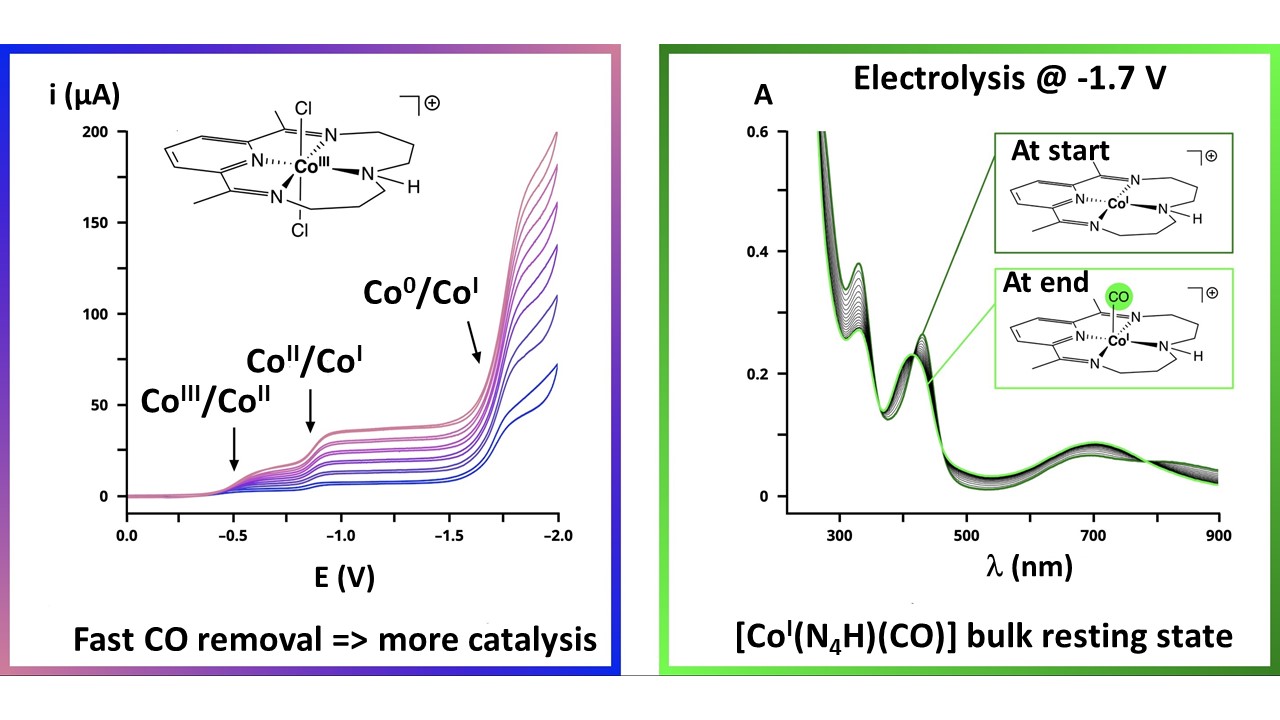
A Self-Moderation Mechanism in CO2 Electroreduction Catalyzed by a Cobalt Macrocyclic Complex.
D. M. Feldman, P.-G. Julliard, J.-C. Madrigalejo, J. Fortage, M.-N. Collomb*, C. Costentin*
J. Am. Chem. Soc, 2026, 148, XXXX-XXXX
10.1021/jacs.5c19804
Published as part of the Special Collection Physical Organic Chemistry (Guest Editors: J. Maddaluno, M. Sebban, S. Lakhdar, and G. Berionni)
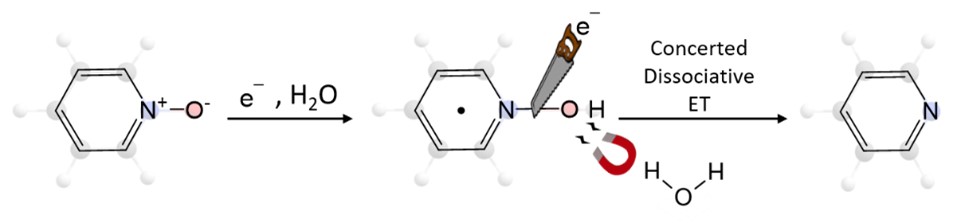
Proton-Coupled Electron Transfer Deoxygenation of Pyridine N-Oxide: A Mechanistic Study.
C. Naddour, G. Durin, S. Chardon-Noblat, C. Costentin*
ChemPhysChem, 2025, 00, e202500292
10.1002/cphc.202500292
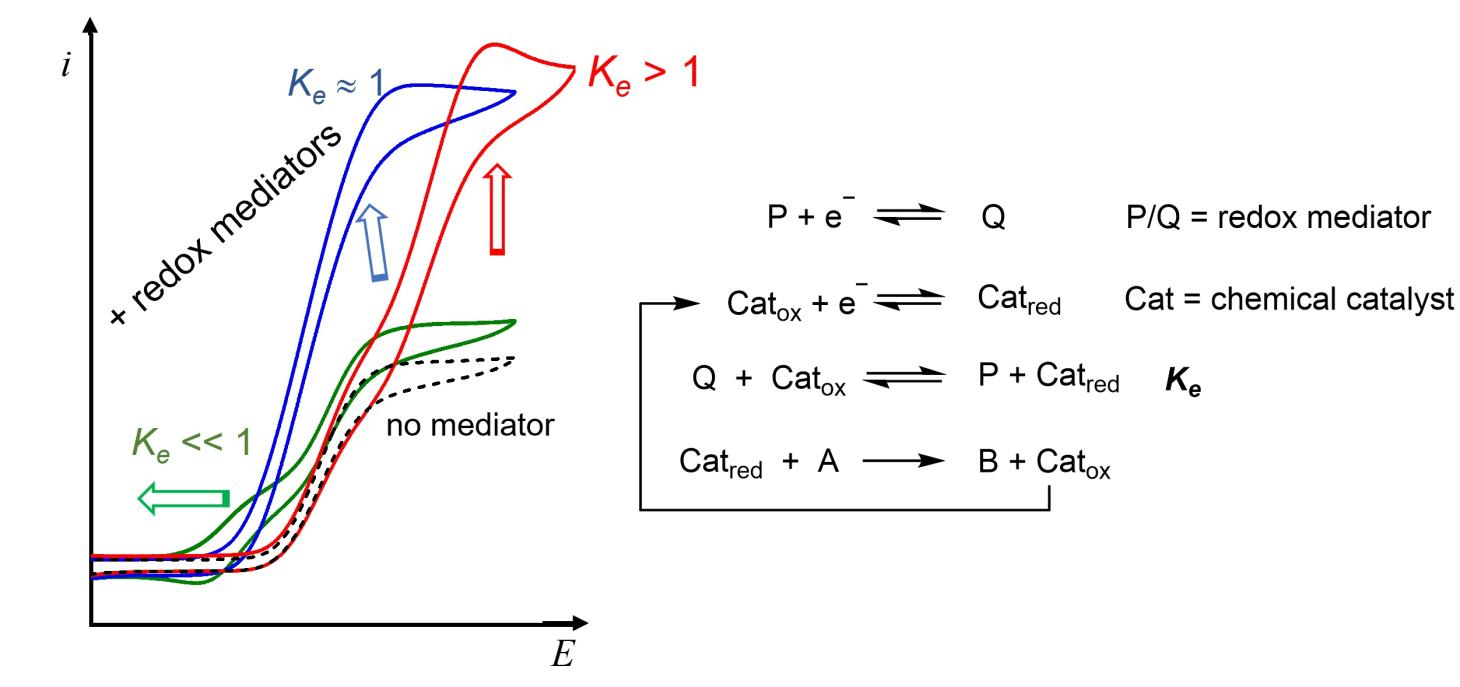
Redox Mediated Molecular Chemical Catalysis of Electrochemical Reactions: A Co-Catalytic Boosting Effect in CO2 Electroreduction Catalyzed by an Iron Porphyrin.
C. M. Harvey, C. Costentin*
ACS Catal., 2025, 15, 14927-14937
10.1021/acscatal.5c03381
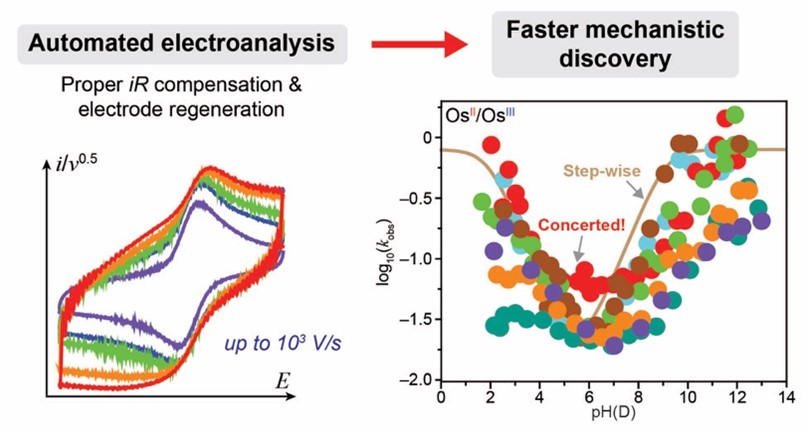
Automated Electroanalysis Accelerates the Discovery of Concerted Proton-Electron Transfer.
J. Sun, Y-A. Lai, J. Sun, Y. Du, B. J. Jolly, H. Sheng, T. Y. Lai, H. M. Chen, C. Costentin, M. Nava*, C. Liu*
J. Am. Chem. Soc., 2025, 147, 28993-29002
10.1021/jacs.5c07228

Efficient Visible-Light-Driven Hydrogen Production in Aqueous Media by the Association of the Triazatriangulenium (TATA+) Dye and the DuBois' Nickel Catalyst.
S. Lyu, D. H. Cruz Neto, J. Aguirre-Araque, L. Termeau, F. Camara, S. Cherraben, D. Martin, E. Brémond, T. Pino, M-H. Ha-Thi, P. P. Lainé*, M.-N. Collomb*, J. Fortage*
Artif. Photosynth., 2025, 1, 251-266
10.1021/aps.5c00013

Published as part of Inorganic Chemistry special issue “Proton-Coupled Electron Transfer in Coordination Chemistry”.
H-Bond Assistance and Electronic Effects to Accelerate the Cleavage of N-O Bond in the Electrochemical Reduction of N2O Catalyzed by Rhenium Bipyridine Tricarbonyl Complexes.
C. Naddour, R. Deeba, M. Kjellberg, S. Chardon-Noblat*, C. Costentin*
Inorg. Chem., 2025, 64, 15217-15223
10.1021/acs.inorgchem.5c02395
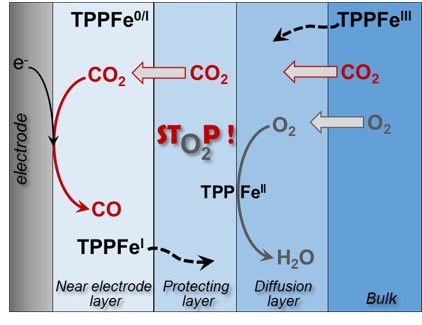
Self-Protection Mechanism and Mass Transport Governing O2 Tolerance in an Iron Porphyrin Homogeneous Catalyst for CO2 Electroreduction.
C. M. Harvey, S. Chardon-Noblat, C. Costentin*
J. Am. Chem. Soc., 2025, 147, 24171-24178
10.1021/jacs.5c09840
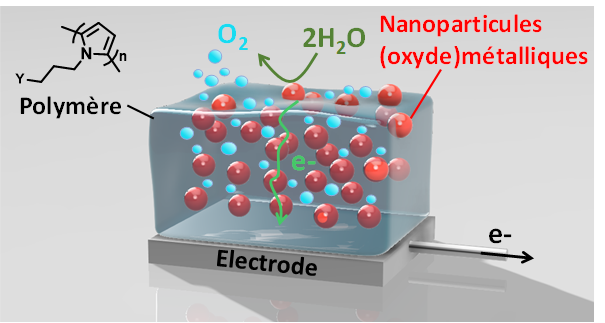
Nanostructuration de catalyseurs métalliques dans un film polymère: vers des matériaux d'électrodes stables et performants.
M.-N. Collomb, J. Fortage, B. Dautreppe, R. Barré, D. Mouchel dit Leguerrier
Actualité Chimique, 2025, 504, 19-24
10.63133/scf.act-chim.2025.504.03
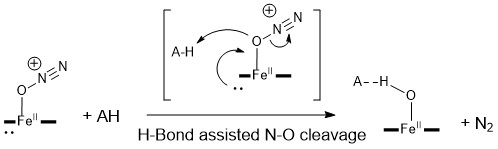
H-Bond Assisted Cleavage of N-O Bond in the Electrochemical Reduction of N2O Catalyzed by Iron Tetraphenylporphyrin.
C. Naddour, R. Deeba, C. Chartier, E. Nicolas, S. Chardon-Noblat, C. Costentin*
ACS Catal., 2025, 15, 9393-9400
10.1021/acscatal/5c01965
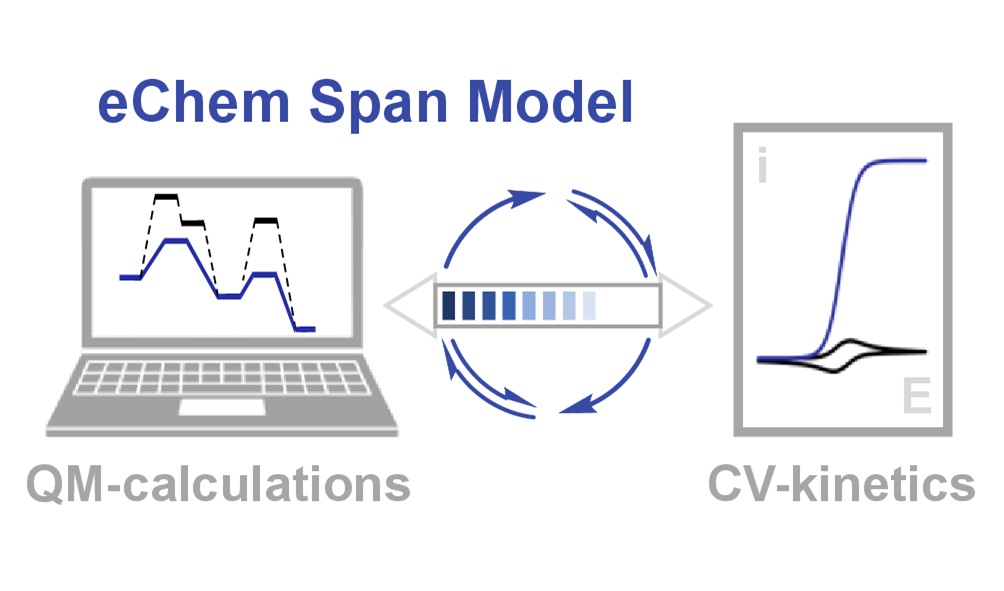
Experiment-Theory Synergy: Connecting the Kinetics of Molecular Catalysis of Electrochemical Reactions with Calculated Energy Landscapes.
G. Durin, C. Costentin*
ACS Catal., 2025, 15, 2501-2514
10.1021/acscatal/4c06976
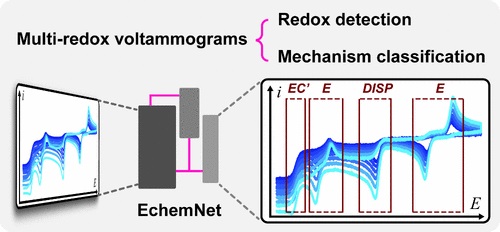
Redox-Detecting Deep Learning for Mechanism Discernment in Cyclic Voltammograms of Multiple Redox Events.
B. B. Hoar, W. Zhang, Y. Chen, J. Sun, H. Sheng, Y. Zhang, Y. Chen, J. Yang, C. Costentin*, Q. Gu*, C. Liu*
ACS Electrochem., 2025, 1, 52-62
10.1021/acselectrochem.4c00014
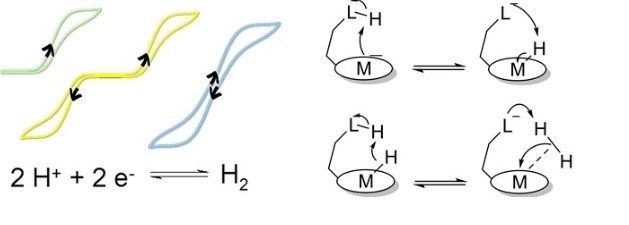
Proton Relays in Molecular Catalysis for Hydrogen Evolution and Oxidation: Lessons From the Mimicry of Hydrogenases and Electrochemical Kinetic Analyses.
M. Haake, B. Reuillard, M. Chavarot-Kerlidou, H, C. Costentin*, V. Artero*
Angew. Chem. Int. Ed., 2024, e202413910
10.1021/anie-202413910
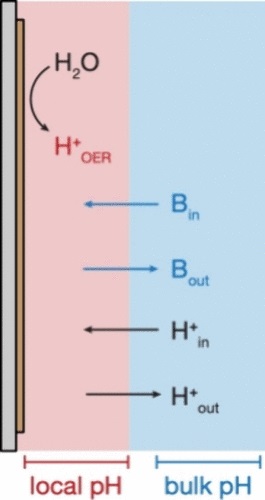
A Straightforward Model for Quantifying Local pH Gradients Governing the Oxygen Evolution Reaction.
S. S. Veroneau, A. C. Hartnett, J. Ryu, H. Hong, H, C. Costentin*, D. G. Nocera*
J. Am. Chem. Soc., 2024, 146, 28925-28931
10.1021/jacs-4c09521
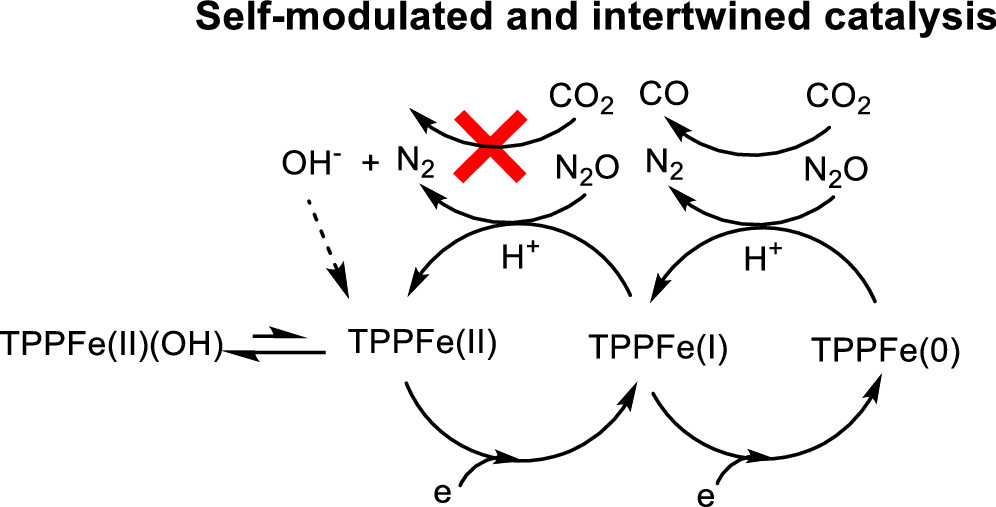
Iron(I) Tetraphenylporphyrin Is an Active Catalyst in Reductive Deoxygenation When Switching from CO2 to Isoelectronic N2O.
C. Chartier, R. Deeba, A. Collard, S. Chardon-Noblat, C. Costentin*
ACS Catal., 2024, 14, 14509-14516
10.1021/acscatal.4c05259
Mechanism of Electrochemical Proton Reduction Catalyzed by a Cobalt Tetraaza Schiff Base Macrocyclic Complex: Ligand Protonation and/or Influence of the Chloro Ligand.
M. Willery, P.-G. Julliard, F. Molton, F. Thomas, J. Fortage*, C. Costentin*, M.-N. Collomb*
ACS Catal., 2024, 14, 11352-11365
10.1021/acscatal.4c0361
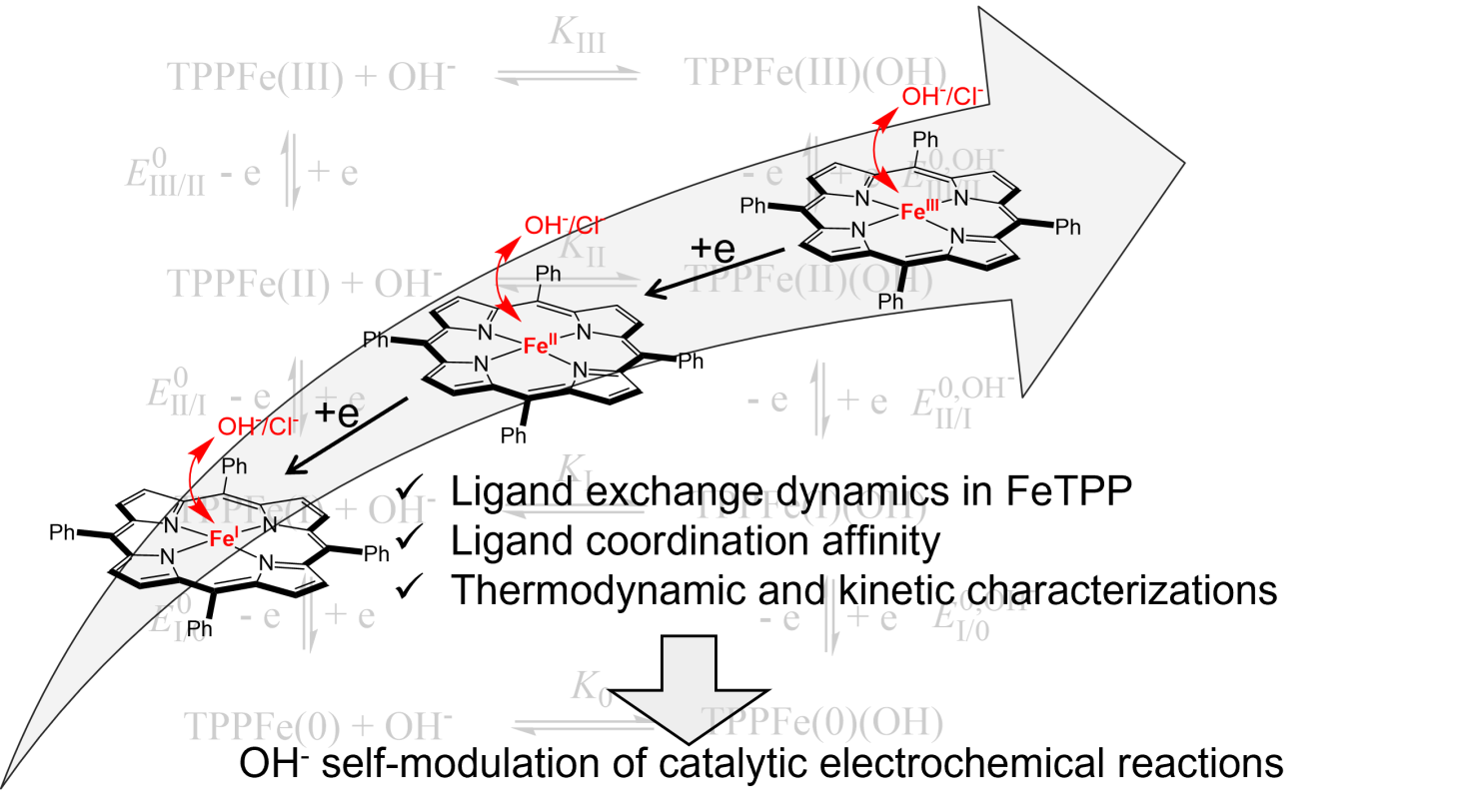
Redox Behavior and Kinetics of Hydroxo Ligand Exchange on Iron Tetraphenylporphyrin: Comparison with Chloro Exchange and Consequences for its Role in Self-Modulation of Molecular Catalysis of Electrochemical Reactions.
C. Chartier, S. Chardon-Noblat, C. Costentin*
Inorg. Chem., 2024, 63, 7541-7548
10.1021/acs.inorgchem.4c00825
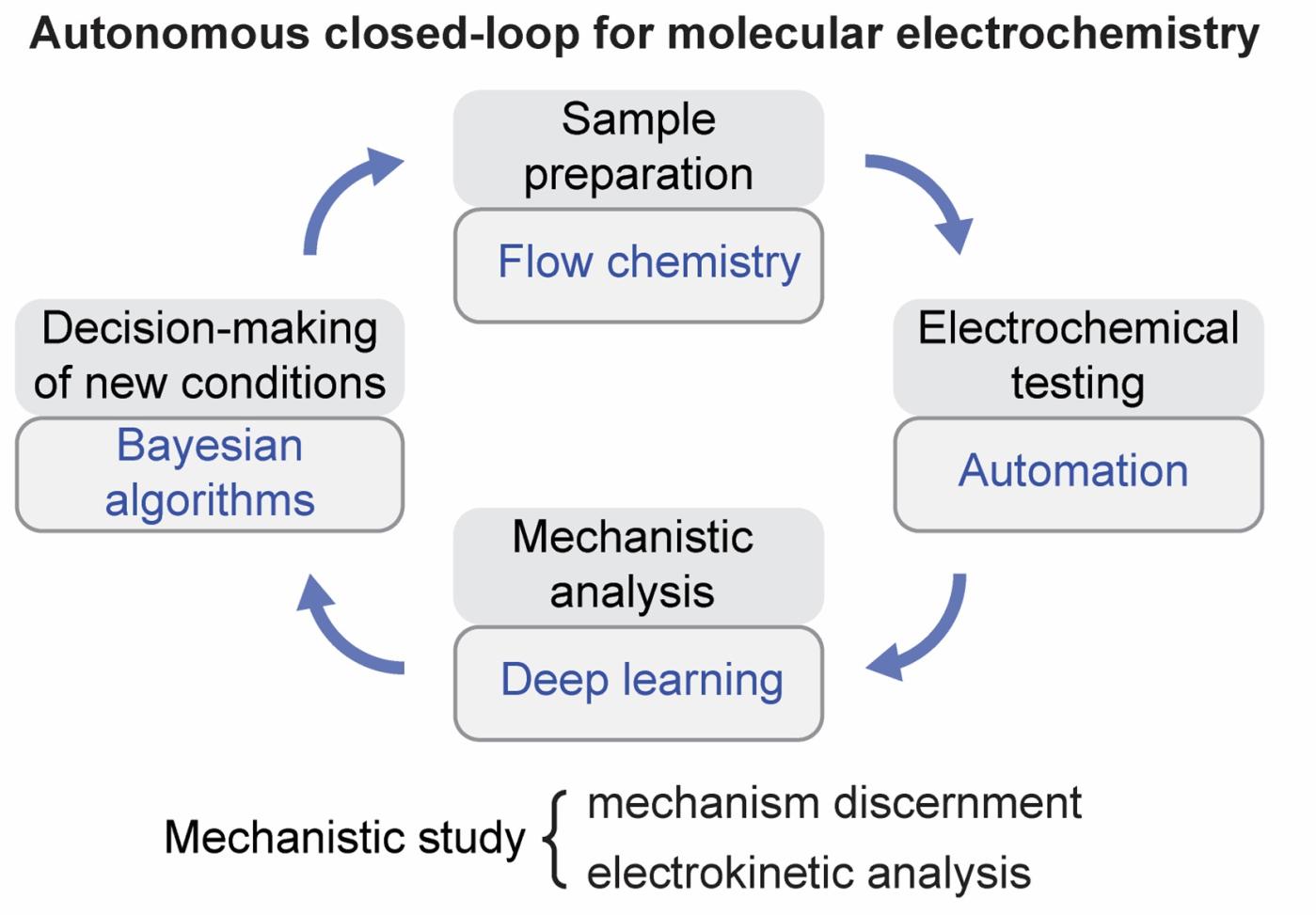
Autonomous closed-loop mechanistic investigation of molecular electrochemistry via automation.
H. Sheng, J. Sun, O. Rodriguez, B. B. Hoar, W. Zhang, D. Xiang, T. Tang, A. Hazra, D. S. Min, A. G. Doyle, M. S. Sigman, C. Costentin, Q. Gu, J. Rodriguez-Lopez, C. Liu*
Nat. Commun., 2024, 15, 2781-2791
10.1038/s41467-024-47210-x
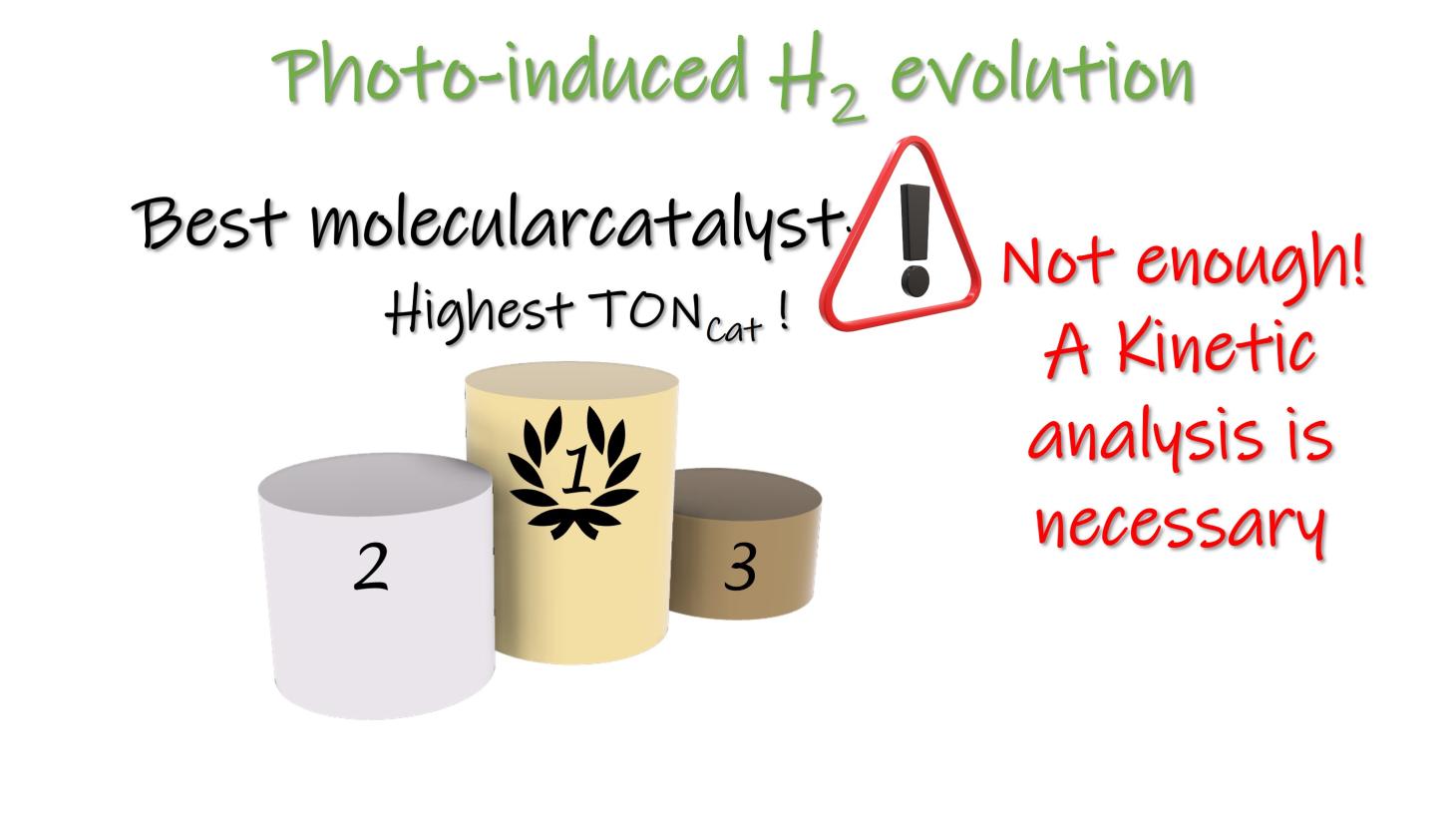
Turnover Number in Photoinduced Molecular Catalysis of Hydrogen Evolution: a Benchmarking of Catalysts?
J. Fortage, M. N. Collomb, C. Costentin*
ChemSusChem, 2024, 0202400205
10.1002/cssc.202400205
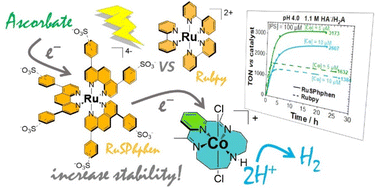
Enhancing the stability of photocatalytic systems for hydrogen evolution in water by using a tris-phenyl-phenanthroline sulfonate ruthenium photosensitizer.
F. Camara, J. S. Aguirre-Araque, J. Fortage*, M-N. Collomb*
Sustainable Energy Fuels, 2024, 8, 1457-1472
10.1039/d3se01556d
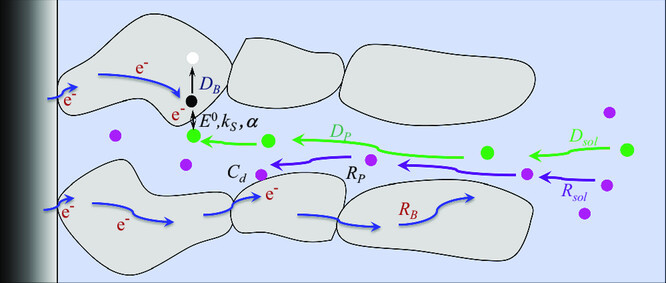
Cyclic Voltammetry to Study Dynamics of Ion Insertion in Porous Materials.
C. Costentin*
Adv. Energy Sustainability Res., 2023, 2300242
10.1002/aesr.202300242
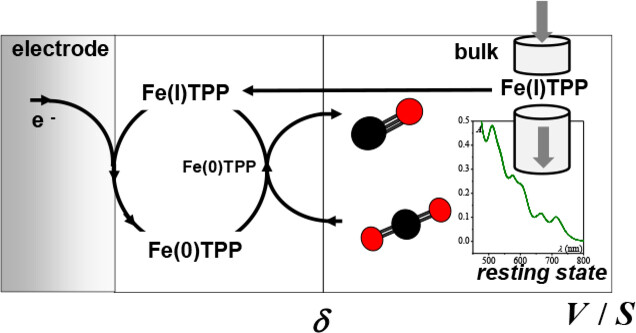
Controlled Potential Electrolysis: Transition from Fast to Slow Regimes in Homogeneous Molecular Catalysis. Application to the Electroreduction of CO2 Catalyzed by Iron Porphyrin.
R. Deeba, A. Collard, C. Rollin, F. Molton, S. Chardon-Noblat, C. Costentin*
ChemElectroChem, 2023, e202300350
10.1002/celc.202300350
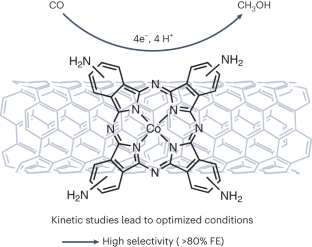
Hybrid catalyst to the rescue.
C. Costentin*
Nat. Synth., 2023
10.1038/s44160-023-00391-7

Importance of Ligand Exchange in the Modulation of Molecular Catalysis: Mechanism of the Electrochemical Reduction of Nitrous Oxide with Rhenium Bipyridyl Carbonyl Complexes.
R. Deeba, S. Chardon-Noblat*, C. Costentin*
ACS Catal., 2023, 13, 8262-8272
10.1021/acscatal.3c01495

Deciphering Reversible Homogeneous Catalysis of the Electrochemical H2 Evolution and Oxidation: Role of Proton Relays and Local Concentration Effects.
B. Reuillard, C. Costentin*, V. Artero*
Angew. Chem. Int. Ed., 2023, e202302779
10.1002/anie.202302779
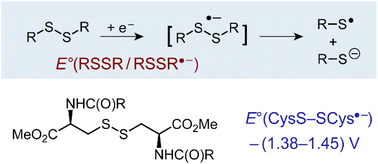
Disulfide radical anion as a super-reductant in biology and photoredox chemistry.
Q. Zhu*, C. Costentin, J. Stubbe, D. G. Nocera*
Chem. Sci., 2023, 14, 6876-6881
10.1039/D3SC01867A
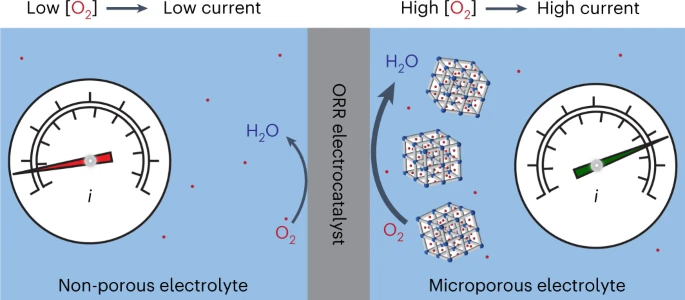
Enhanced activity for the oxygen reduction reaction in microporous water.
A. E. Thorarinsdottir, D. Erdosy, C. Costentin*, J. A. Mason*, D. G. Nocera*
Nat. Catal., 2023, 6, 425-434
10.1038/s41929-023-00958-9
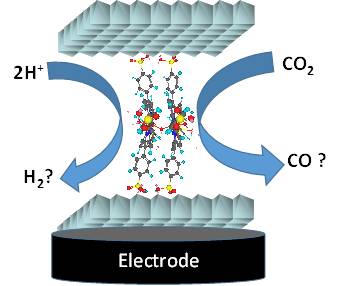
Behavior of Iron Tetraphenylsulfonato Porphyrin Intercalated into LDH and LSH as Materials for Electrocatalytic Applications.
A. Tarhini, J. Aguirre-Araque, M. Guyot, C. Costentin, R. Rogez*, S. Chardon-Noblat*, V. Prevot, C. Mousty*
Electrocatalysis, 2023, 14, 111-120
10.1007/s12678-022-00778-8
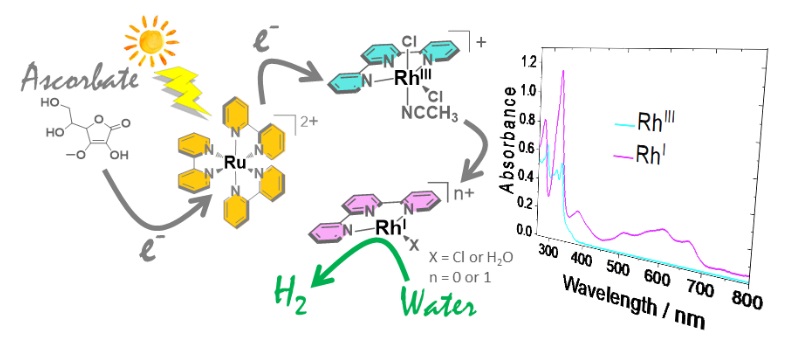
Electrochemical properties of a Rhodium(III) mono-terpyridyl complex and use as a catalyst for light-driven hydrogen evolution in water.
F. Camara,T. Gavaggio B. Dautreppe, J. Chauvin, J. Pécaut, D. Aldakov, M.-N. Collomb*, J. Fortage*
Molecules, 2022, 27(19), 6614
10.3390/molecules27196614
hal.archives-ouvertes.fr/hal-03808904
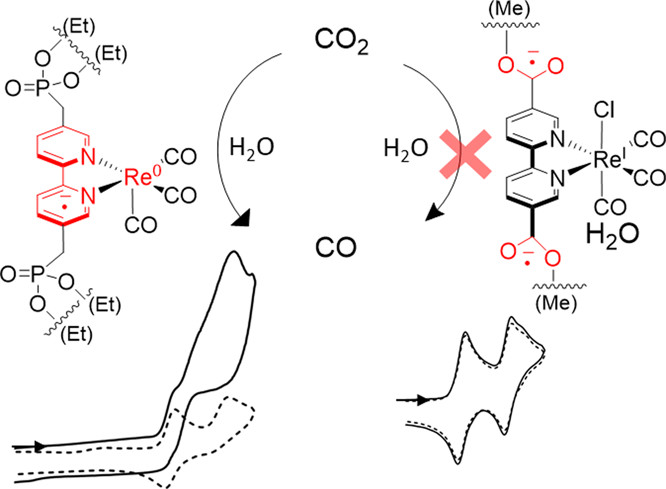
Effect of Substituents Mimicking Anchorage of Rhenium Carbonyl Bipyridine Molecular Catalysts on CO2 Electroreduction
M. Guyot, M-N. Lalloz, J. S. Aguirre-Araque, G. Rogez, C. Costentin*, S. Chardon-Noblat*
Inorg. Chem., 2022, 61, 16072-16080
10.1021/acs.inorgchem.2c02473
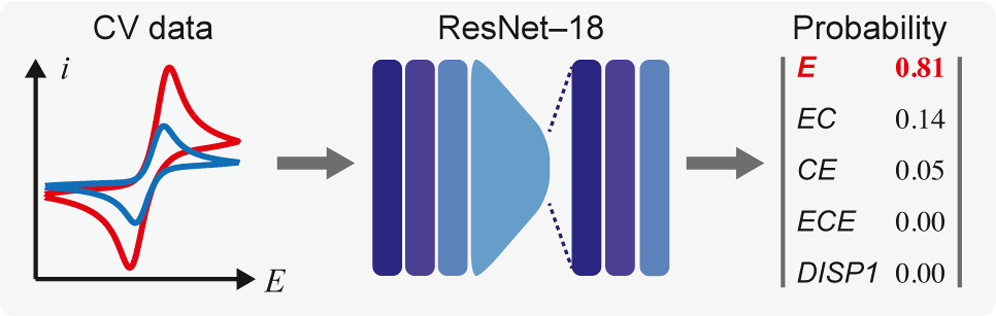
Electrochemical Mechanistic Analysis from Cyclic Voltammograms Based on Deep Learning.
B. B. Hoar, W. Zhang, S. Xu, R. Deeba, C. Costentin*, Q. Gu*, C.Liu*
ACS Meas. Sci. Au, 2022, 2, 55-604
10.1021/acsmeasuresciau.2c00045
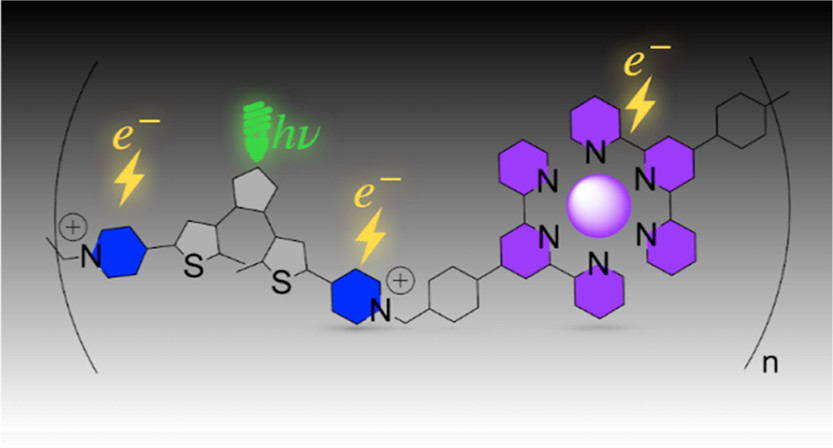
Photochromic Metallopolymer Based on Dithienylethene as a Molecular Calculator.
E. Chatir, A. Khettabi, F. Lafolet, D. Jouvenot, G. Royal, E. Saint-Aman, S. Cobo*
Chem. Mater., 2022, 34, 5912-5918
10.1021/acs.chemmater.2c00819
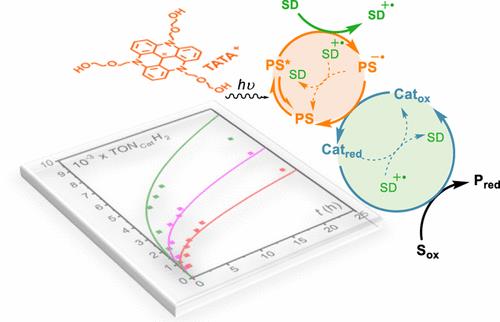
Photoinduced Catalysis of Redox Reactions, Turnover Numbers, Turnover Frequency and Limiting Processes: Kinetic Analysis and Application to Light-Driven Hydrogen Production.
C. Costentin*, F. Camara, J. Fortage, M.-N. Collomb
ACS Catal.. 2022, 12, 6246-6254.
10.1021/acscatal.2c01289
hal.archives-ouvertes.fr/hal-03766011
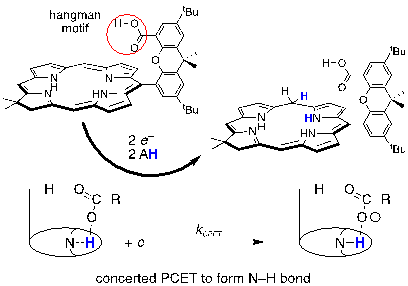
Proton Coupled Electron Transfer of Macrocyclic Ring Hydrogenation: The Chlorinphlorin.
R. Sun, M. Liu, S-L. Zheng, K. D. Dogutan*, C. Costentin*, D. G. Nocera*
Proc.Natl. Acad. Sci USA, 2022, 119, e2122063119
10.1073/pnas.2122063119
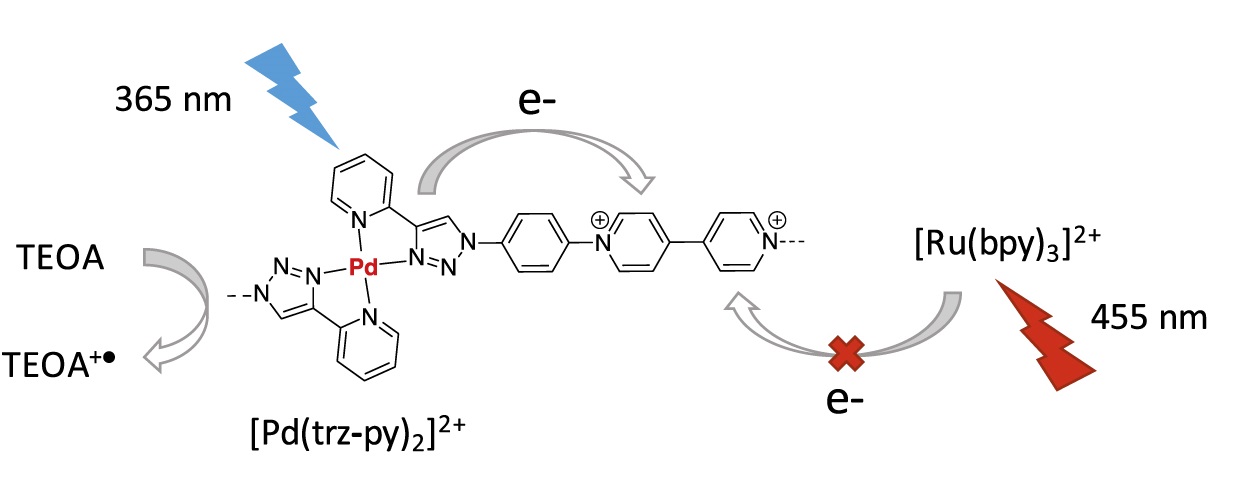
Photoredox Processes in the Aggregation and Gelation of Electron- Responsive Supramolecular Polymers Based on Viologens
C. Roizard, V. Andrieux, S. Al Shehimy, S. Chowdhury, Q. Reynard-Feytis, C. Kahlfuss, E. Saint-Aman, F. Chevallier, C. Bucher*, T. Gibaud*, D. Frath*
ECS Adv., 2022, 1, 020502
10.1149/2754-2734/ac6ad4
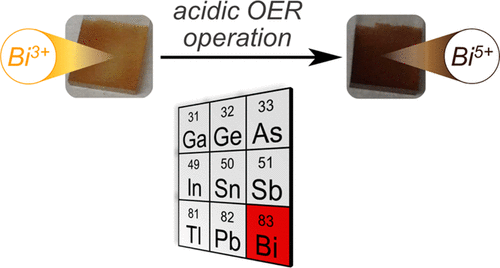
p-Block Metal-Oxide Noninnocence in the Oxygen Evolution Reaction in Acid: The Case of Bismuth Oxide.
A. E. Thorarinsdottir, C. Costentin, S. S. Veroneau, D. G. Nocera*
Chem. Mater., 2022, 34, 826-835
10.1021/acs.chemmater.1c03801
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn