- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
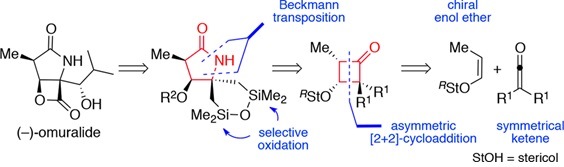
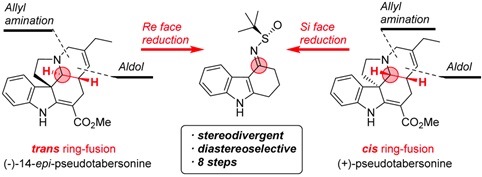
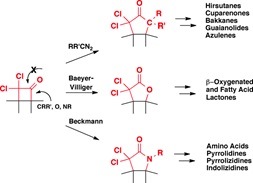
We are interested in the synthesis of alkaloid natural compounds, widely present in nature and offering an endless source of inspiration and creativity. Currently, we are focusing our attention on the lycorine family, salinosporamide derivatives, ß-substituted α-amino acids, ketene chemistry, and reactivity of thiofunctionalized compounds. Some of these natural products are isolated in unusable yields (small quantities) and often have an incorrect chemical reported structure. In our synthetic studies, we always wish to develop new chemical transformations (or methodologies) and offer efficient and original ways for their synthesis and characterization, but also to allow the access to analogs.
Selected publications
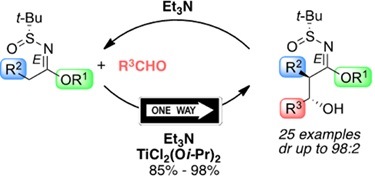
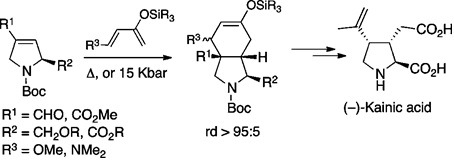
Orellana, A., Pandey, S. K., Carret, S., Greene, A. E., & Poisson, J. F. (2012). A Diels-Alder-Based Total Synthesis of (-)-Kainic Acid. Journal of Organic Chemistry, 77 (12), 5286-5296. doi:10.1021/Jo300608g
Pandey, S. K., Orellana, A., Greene, A. E., & Poisson, J. F. (2006). High-pressure Diels-Alder approach to natural kainic acid. Organic Letters, 8 (24), 5665-5668.doi:10.1021/ol062419l
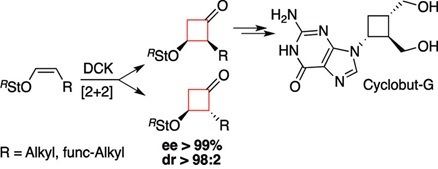
Darses, B., Greene, A. E., & Poisson, J. F. (2012). Asymmetric Synthesis of Cyclobutanones: Synthesis of Cyclobut-G. Journal of Organic Chemistry, 77 (4), 1710-1721. doi: 10.1021/jo202261z
Darses, B., Greene, A. E., Coote, S. C., & Poisson, J. F. (2008). Expedient approach to chiral cyclobutanones: Asymmetric synthesis of cyclobut-G. Organic Letters, 10 (5), 821-824. doi:10.1021/ol70977x
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn