- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
Known for over a century, ketenes are unique reactive species due to their original cumulenic structure. Ketene cycloadditions followed by ring expansion have been exploited in our group to achieve the total synthesis of many natural products. But our interest is also focused on mechanistic studies, on developing an unusual (3+2) cycloaddition and on exploring flow chemistry.
Selected publications
Rullière, P., S. Carret, A. Milet, and J-F. Poisson, "Kinetic Resolution in the [2+2] Cycloaddition of Ketenes: An Experimental and Theoretical Study.", Chem. Eur. J., vol. 21, no. 10: Wiley-VCH Verlag GmbH {&} Co. KGaA, pp. 3876–3881, 2015.
Depres, J-P., P. Delair, J-F. Poisson, A. Kanazawa, and A. E. Greene, "Diverse Natural Products from Dichlorocyclobutanones: An Evolutionary Tale.", Acc. Chem. Res., vol. 49, no. 2: American Chemical Society, pp. 252–261, 2016.
Rullière, P., J. Grisel, C. Poittevin, P. Cividino, S. Carret, and J-F. Poisson, "Thermal [2 + 2]-Cycloaddition of Ketenes with Chiral Enol Ethers: Route to Densely Substituted Cyclobutanones.", Org. Lett., vol. 18, no. 12: American Chemical Society, pp. 2824–2827, 2016.
Viceriat, A., I. Marchand, S. Carret, and J-F. Poisson, "Synthesis of gamma-Lactams by Formal Cycloadditions with Ketenes", Org. Lett., vol. 23, no. 7: American Chemical Society, pp. 2449-2454, 2021.
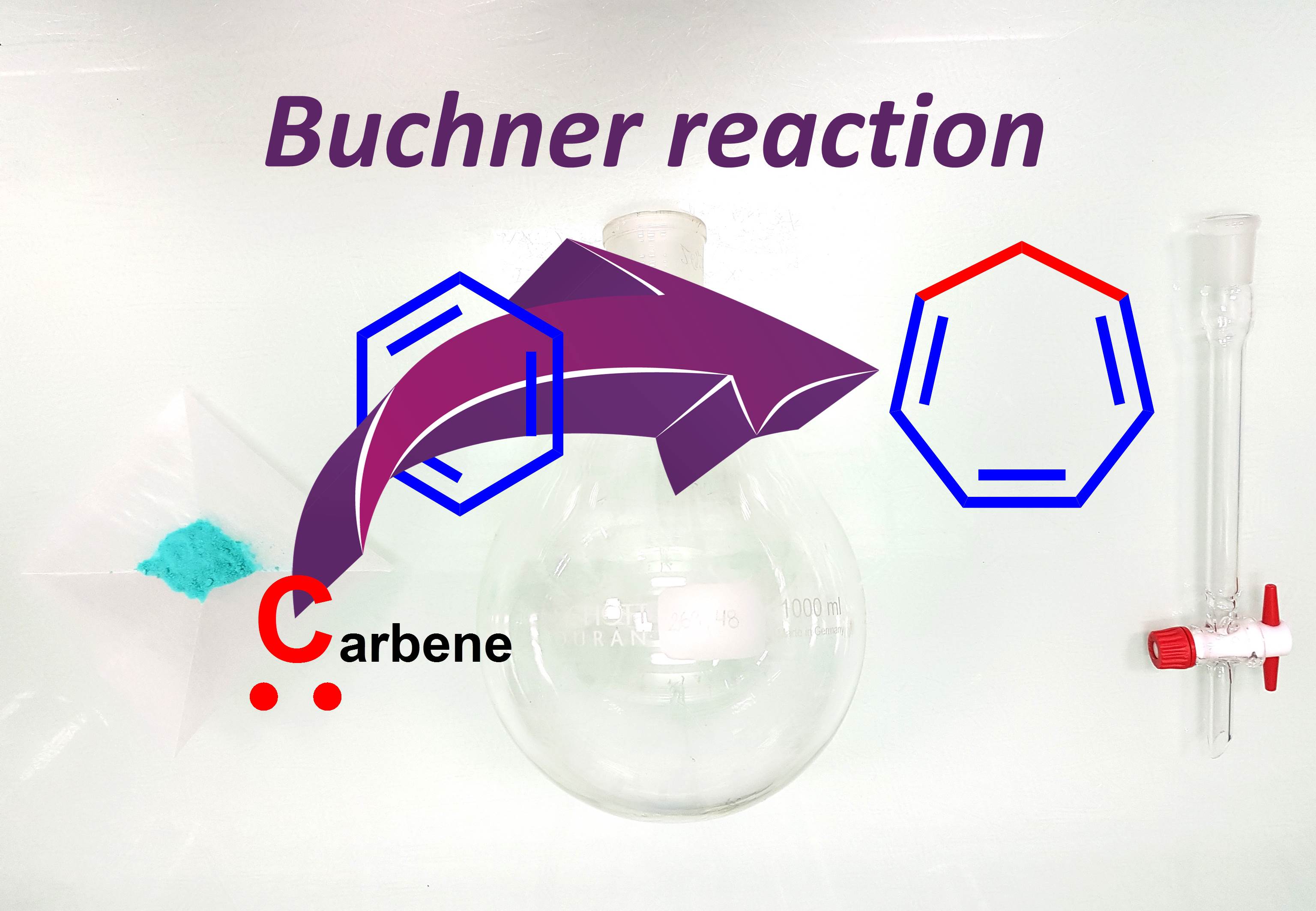
Carbenes are reactive species able to undergo various insertion reactions, like the cyclopropanation of aromatic rings. This original neutral dearomatization method is named the Buchner reaction. We recently turned our attention toward the catalytic version of this transformation which delivers original cycloheptatriene-containing structures present in various synthetic targets, and we study the selectivity of the reaction.
Selected publications
Darses, B., P. Maldivi, C. Philouze, P. Dauban, and J-F. Poisson, "Asymmetric Intramolecular Buchner Reaction: From High Stereoselectivity to Coexistence of Norcaradiene, Cycloheptatriene, and an Intermediate Form in the Solid State", Org. Lett., vol. 23, no. 2: American Chemical Society, pp. 300-304, 2021.
Phung, B. T., Medini, A., Maldivi, P., Poisson, J-F., Bessières, B., Darses, B., "Intramolecular Buchner Reaction: Experimental and Theoretical Comparison of the Effect of the Carbene Substituent on the Chemoselectivity", Adv. Synth. Catal., vol. 366, no. 20: Wiley, pp. 4211-4218, 2024.
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
