- Imprimer
- Partager
- Partager sur Facebook
- Share on X
- Partager sur LinkedIn
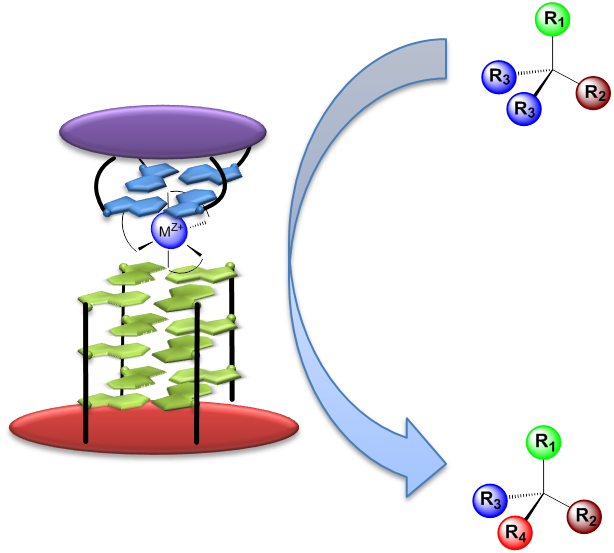
Both public demand and government regulations urge manufacturers to develop environmentally improved enantioselective routes for the production of fine chemicals and pharmaceuticals. Replacement of stoichiometric reagents for synthetic reactions with catalytic routes is one of the most efficient methods to develop greener, safer, and more cost-effective chemical processes.
In this context, we propose to develop new enantioselectives catalysts based on the association of 1- oligonucleotides which is structurally constrained (e.g. Template Assisted Synthesis of G-Quadruplex” ); 2- a metal complex which has demonstrated to be efficient to develop artificial metalloenzymes (in collaboration with LCBM, Grenoble) and 3- a synthetic G-tetrad (in collaboration with ICUMB, Dijon).
For non-enantioselective peroxidation reactions, we have demonstrated that the association of TASQ and hemin is a competent catalyst (Nanoscale 2014) and that a synthetic G-tetrad can boost this reaction (Chem. Eur. J. 2016).
We propose to extend this concept to develop new enantioselective catalysts
Students and post-docs are encouraged to contact us for research opportunities.
This project is supported par ANR (n° ANR-18-CE07-0017, CoolCat).
Recent collaborations:
C. Marchi-Delapierre, S. Ménage (LCBM, CEA Grenoble) for metallo-enzyme catalysis
D. Monchaud (ICMUB, Dijon) for G-tetrad boosting
Project Staff
- Imprimer
- Partager
- Partager sur Facebook
- Share on X
- Partager sur LinkedIn