- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
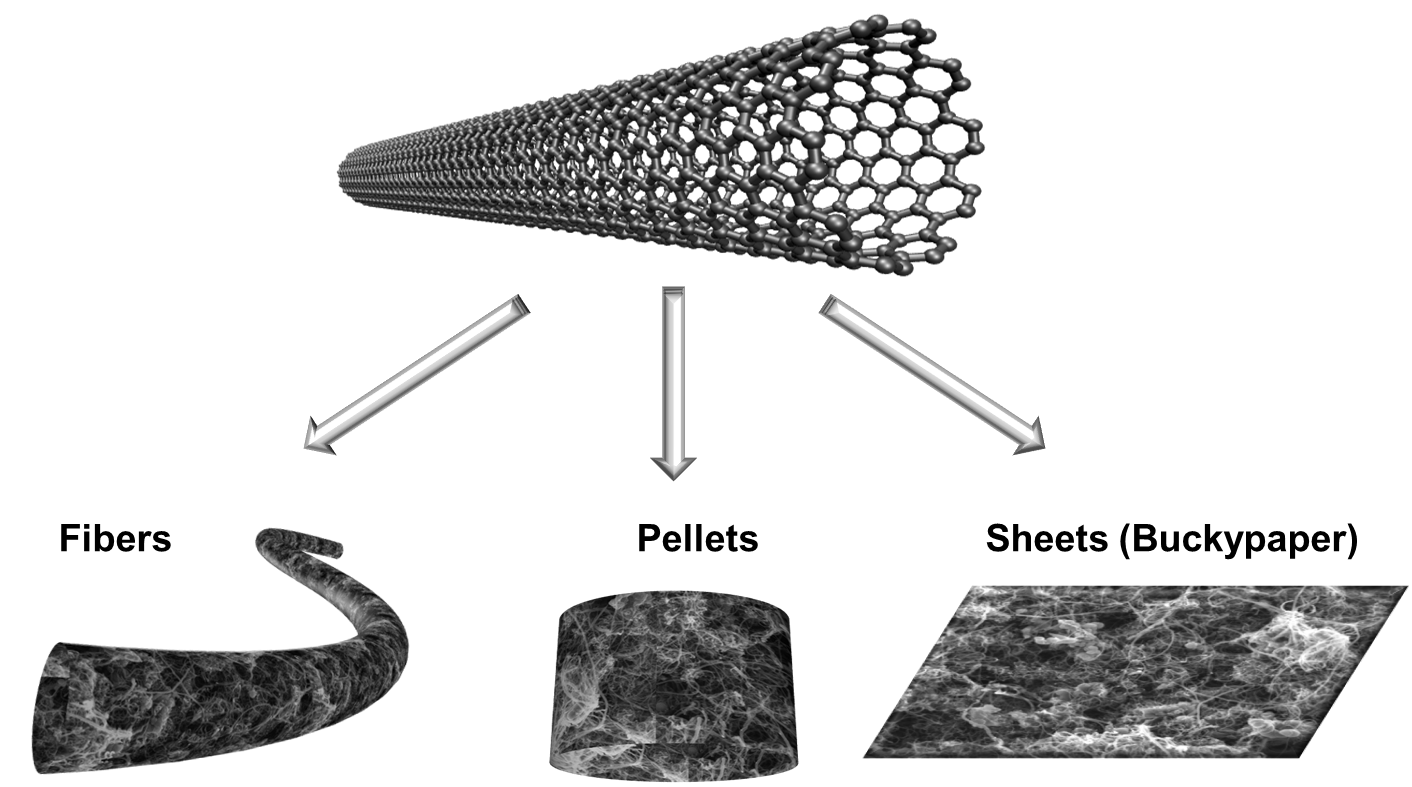
Carbon nanotubes (CNTs) have very favored properties as electrode material for the electron transfer with enzymes due to their nanowire structures enabling naturally close contact to the redox centers of some enzymes (more). CNTs can further be shaped to give free-standing electrodes which facilitates the integration on electronic devices (more)
Buckypapers
Buckypapers are sheets of randomly oriented and entangled CNTs and are the most prominent example of shaped CNTs. The origin of this terminology goes back to the discovery of Fullerenes in 1985. These football structured molecules were named in memory of the famous architect Buckminster Fuller. Fullerenes were also called Buckyballs as some kind of nickname. When CNTs were identified in the 90’s, they were initially described as nanotubules or Buckytubes since they were initially considered as tubular extension of fullerenes. The possibility to form paper like sheets with CNTs led to the term “Buckypapers” which remained until now a well-accepted term for such CNT sheets.
Buckypapers are usually formed by simple vacuum filtration of a CNT dispersion where the mechanic stability depends on the quality of the CNTs and their dispersion. Buckypaper can also be produced via rolling methods, such as “domino pushing” and CNT drawing and winding.
A sophisticated approach for Buckypapers based biocathodes was developed in pour team using ABTS as mediator (more). Here, a redox active BP was formed using a bispyrene modified ABTS. The two pyrene units on this mediator act as cross-linker for the reinforcement of the formed Buckypapers obtained by vacuum filtration of a CNT- ABTS bis-pyrene mixture. Furthermore, laccase from Trametes versicolor has a hydrophobic domain close to one of the four copper centers which supplies the three other copper ions with electrons to reduce oxygen to water. With this hydrophobic region, the enzyme can be immobilized and oriented with small hydrophobic molecules like anthraquinone (more) or pyrene (more) enabling DET with the electrode material. For the bis-pyrene-ABTS Buckypapers , by saturating the CNT surface with the pyrene modified ABTS, a high amount of free pyrene groups is available for the anchoring and orientation of laccase. More interesting, the redox mediator Buckypapers allows efficient electron transfer even when the enzyme is in solution. This represents a promising advantage since enzymes in solution are easier to replace that the whole bioelectrode when the activity of these biocatalysts decreases.
More recently, we have developed a freestanding paper biofuel cell comprising redox-molecule embedded multi-walled carbon nanotube buckypapers for electrical wiring of enzymes. The drop-coat and one-pot fabrication methods provide flexibility and permit easy scalability of functionalised bioelectrodes via commercially available materials. Buckypaper functionalised with 1,10-phenanthroline-5,6-dione (PLQ) as a new electron mediator for fungal-derived FAD-dependent glucose dehydrogenase (FADGDH) shows remarkably high steady-state current densities for glucose oxidation (more).
Pellets
CNT pellets are formed by compression and its shaping was particularly developed for the formation of bioelectrodes used for biofuel cell applications. By compressing CNTs in presence of enzymes and, when needed, with the corresponding mediator, highly performant bioelectrodes can be formed since a huge quantity of enzyme can thus be incorporated inside the CNT matrix. The first pure CNT-enzyme pellet electrode fuel cell was reported by our team (more) by compression of the CNTs in presence of glucose oxidase (GOx) and catalase for the anode and laccase for the cathode. To investigate the potential of this setup to generate power out of body liquids, the bioanode and the biocathode were packed in a sterilized environment to assure biocompatibility. The whole fuel cell and packaging was implanted in the abdomen of a rat generating a power density of almost 200µW cm-2 which was sufficient to flash a LED and to turn a digital thermometer for several minutes (more).
In spite of these promising results, later studies revealed that only a very little amount of enzymes is wired to the CNT matrix at the bioanode. In fact, DET with GOx is still a challenge since the active center is deeply embedded inside the protein structure and the cofactor FAD, responsible for the intrinsic electron transfer, can be released from the enzyme that deactivates its catalytic properties. To improve the bioanode performances, we included in the compression process the redox mediator naphthoquinone which led to a current density increase of more than one magnitude for the electrocatalytic oxidation of glucose (more).
Fibers
CNT fibers can be produced by electrospinning, wet spinning methods, or by drilling of CNT yarns with diameters ranging from several micrometers down to few hundreds of nanometers. Electrospinning is a convenient technique to produce fibers at submicron scale from a large variety of polymers. The process is quite simple and ensures the elaboration of fiber mats with controlled characteristics.
We developed an interesting strategy to wire laccase on electrospun carbon fibers based on polyacrylonitrile (PAN)-CNT fibers. Laccase was immobilized on these fibers by standard EDC amide coupling leading to randomly oriented enzymes. About one third of enzymes already provided DET for the electrocatalytic reduction of oxygen. The measured current densities could almost be doubles by using a molecular plug based on the previously mentioned bis-pyrene-ABTS. One pyrene group forms supramolecular interactions with the hydrophobic domain of Laccase while the other pyrene function adsorbs on the amorphous fiber surface thus establishing MET (more).
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn