- Imprimer
- Partager
- Partager sur Facebook
- Share on X
- Partager sur LinkedIn
Designing efficient and selective catalytic systems remains an important challenge to address current societal issues in the fields of energy, environment or health. Our strategy is to take inspiration from Nature and more particularly from the active sites of sulfur-rich metalloenzymes.
Bio-inspired H2 production
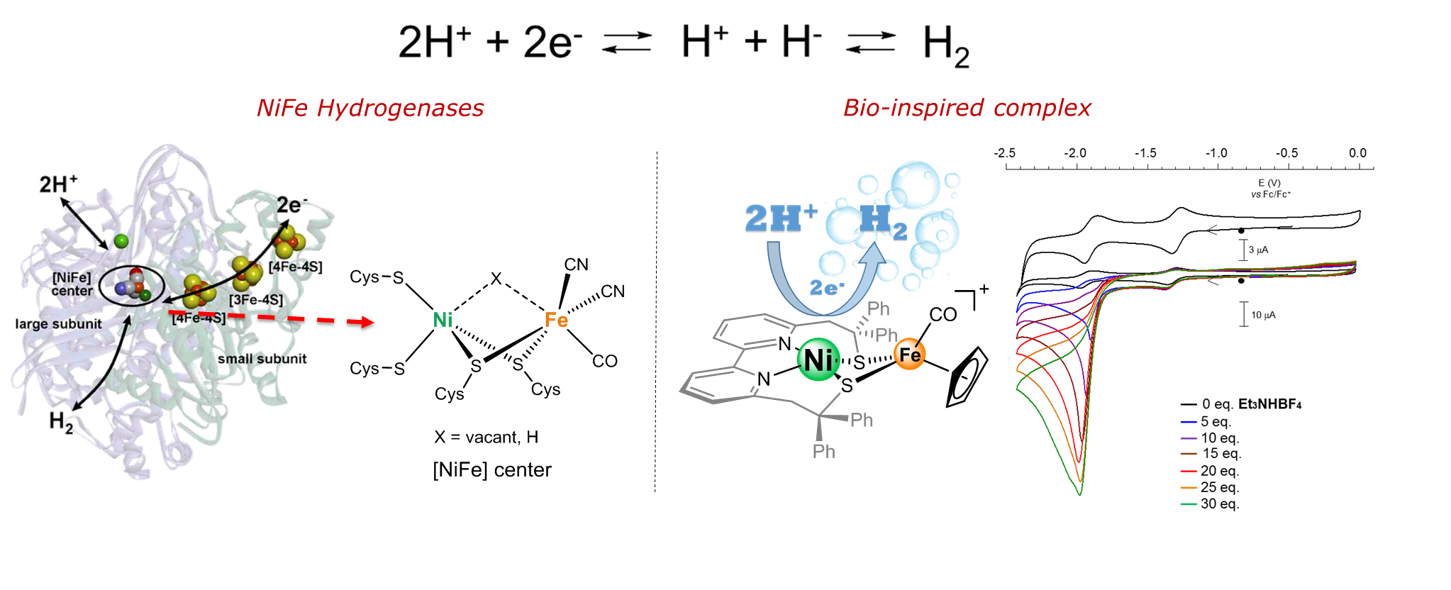
In order to develop efficient catalysts for H2 production, we target transition metal complexes that mimic the active site of [Ni·Fe] hydrogenase. We have synthesized a structural and functional model whose redox reactivity is centered on the nickel center as in the enzyme. We have also detected an intermediate involved in the catalytic H2 production cycle that mimics a [NiFe]-hydrogenase intermediate (Ni-L state). The kinetics of hydrogen production catalyzed by the [NiFe]-mimic unambiguously demonstrates the synergy between nickel and iron in driving the process. A comparative evaluation of the performances of the nickel-iron catalyst reveals that these far surpass those of the other dinuclear biomimetic models.
Catalysts for O2 reduction
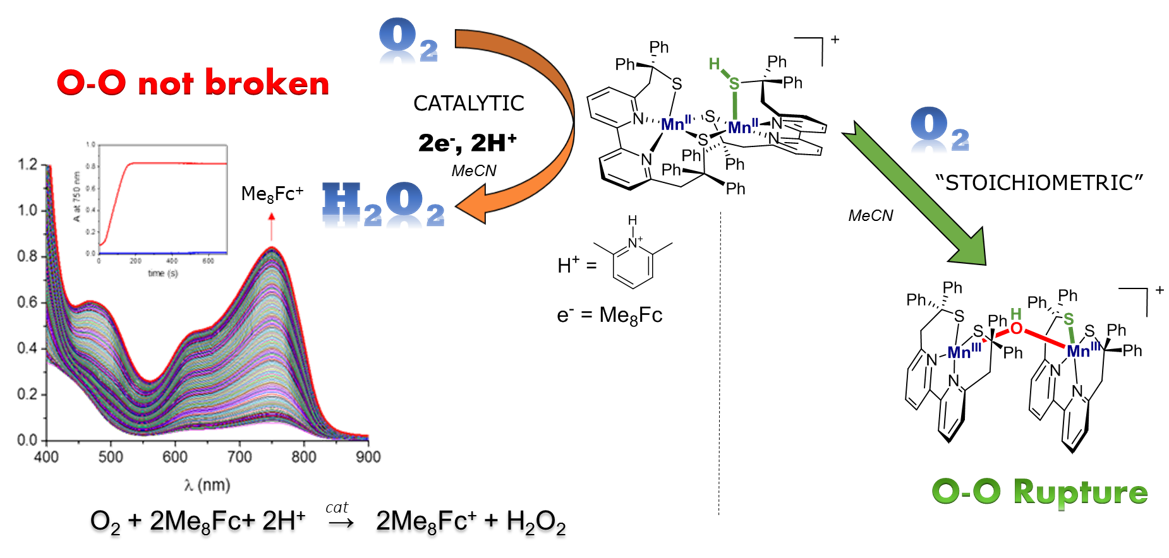
Activating oxygen is a critical step in many biological and industrial processes. The selectivity of O2 reduction is difficult to control: 2- vs. 4-electron reduction togenerate H2O2 vs. H2O, respectively. How is this process performed within metal complexes? What are the parameters that control the reactivity of the metal-oxygen species? These questions remain central to the chemist. Our strategy is to investigate the effect of the experimental conditions on the selectivity and the mechanism including generation and characterization of intermediate species, especially through the study of Mn- and Fe- thiolate complexes.
Collaborations
V. Artero (Grenoble)
F. Meyer (Germany)
S. De Visser (UK)
W. Browne (NL)
M. Hall (USA)
M. Orio (Marseille)
Selected publications
- Imprimer
- Partager
- Partager sur Facebook
- Share on X
- Partager sur LinkedIn