- Imprimer
- Partager
- Partager sur Facebook
- Share on X
- Partager sur LinkedIn
Organic radicals coordinated to transition metals are widely spread in Nature. In biological systems, they act as efficient and powerful catalysts, able to achieve highly selective reactions and finely-tailored organic compounds. Also, those natural catalysts use renewable ressources (earth-abundant metals, O2...) that contribute to reduce waste management (green by-products, atom-economy...).
Taking inspiration from Nature, our research focuses on the design of unique molecular catalysts for the development of sustainable synthetic methodologies.
Radical catalysts and earth-abundant metals
Polydentate architectures derived from anilines and o-phenylenediamine are promising candidates to elaborate bio-inspired catalysts. Indeed, once deprotonated and coordinated to a metal center under aerobic conditions, those ligands give access to stable radical complexes. Our work aims at understanding the reactivity and the catalytic activity of unprecedented species, applied to various synthetic transformations.
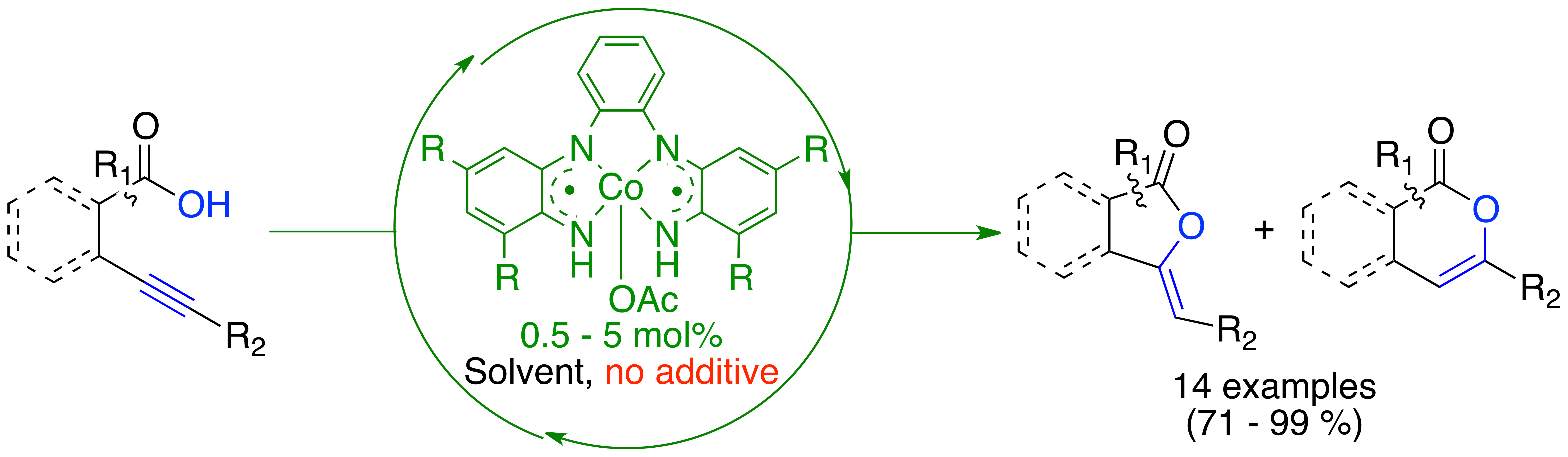
We recently developed the first cobalt catalyzed intramolecular oxy-carbonylation of alkynoic acids, thanks to the design of a unique diradical cobalt(III) complex. The reaction proceeds smoothly, without any additives. The methodology was successfully applied to 14 substrates. The cobalt catalyst is air- and moisture-stable and was found more active than most of the catalytic systems based on noble metals.
O2 as a green oxidant / O-atom source
Polydentate architectures derived from anilines and o-phenylenediamines are under-exploited, mainly due to their facile oxidation. Nevertheless, this feature is particularly attractive for a catalytic application that could benefit from O2. Our efforts are directed towards the design of renewable radical catalysts that could activate and use O2 in oxidation/oxygenation reactions.
- Imprimer
- Partager
- Partager sur Facebook
- Share on X
- Partager sur LinkedIn