- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
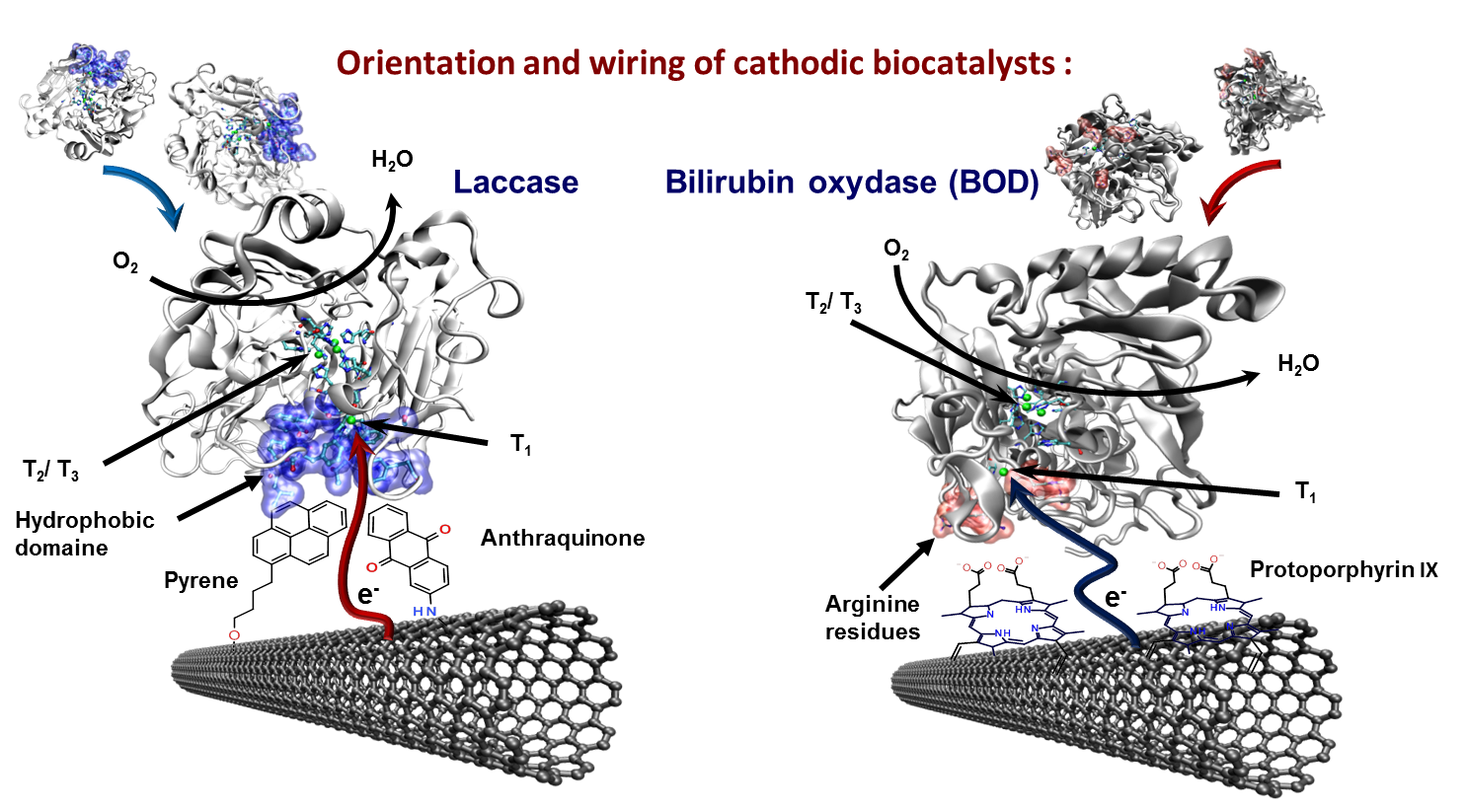
Most enzymes used for the bioelectrocatalytic reduction of oxygen into water are from the multicopper enzyme family where laccases and bilirubin oxidases (BOD) can be considered as enzymes of choice. They are composed of two distinct redox centers where a trinuclear 2 and 3 type (T2/T3) center reduces oxygen to water in a four electron process and a mononuclear 1 type (T1) center usually oxidizes its natural substrate (in general phenolic compounds) in a one electron process and supplies the T2/T3 center with the harvested electrons. These multicopper enzymes are generally smaller than the ones for glucose oxidation and the electron transfer is more evident to achieve since the active sites are more accessible.
Beside the highly efficient approaches for mediated electron transfer for laccases, the fact that the active centers are close to the protein surface motivates to achieve the generally preferred DET. Furthermore, Laccase from Trametes versicolor is particularly suitable for optimized DET wiring since the protein structure provides a hydrophobic domain close to the T1 center.
We reported several examples for the immobilization, orientation, and wiring of laccase using polyaromatic hydrocarbons such as pyrene (see more) or anthraquinone (see more) derivatives attached to carbon surfaces and therein mainly carbon nanotubes. Lalaoui et al. studies this phenomenon in more detail and calculated the binding energy of the anthraquinone representing the polyaromatic compounds and adamantane, a saturated hydrocarbon (see more).
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn