- Imprimer
- Partager
- Partager sur Facebook
- Partager sur Twitter
- Partager sur LinkedIn
- Share url
Parution / Recherche
Le 30 juillet 2018
This themed issue ultimately aims to reflect the strength and vitality of European inorganic chemistry and is guest-edited by Professors Sascha Ott (Uppsala University, Sweden), Richard Layfield (University of Manchester, UK), Marinella Mazzanti (EPFL, Switzerland) and Nils Metzler-Nolte (Ruhr-Universität Bochum, Germany).
Seven-coordinate lanthanide complexes with a tripodal redox active ligand: structural, electrochemical and spectroscopic investigations
Fabrice Thomas et coll.
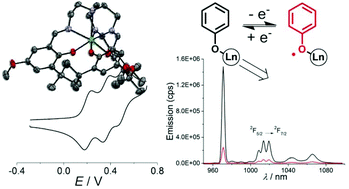
The tripodal ligand TREN-(3,5-di-tert-butylsalicylidene)3 (H3L) was synthesized and its tris(phenolato) lanthanide complexes L-Ln (Ln = NdIII, EuIII, TbIII, GdIII, ErIII, YbIII and LuIII) were prepared. The X-Ray crystal structures confirm that each metal ion resides in a similar monocapped octahedral geometry, excluding water molecules from the coordination sphere. The coordination bond distances are in agree- ment with the lanthanide contraction, with Ln–O bond lengths in the range 2.139–2.216 Å. The com- plexes show three reversible monoelectronic oxidation waves, which are assigned to the successive oxi- dation of the phenolate moieties to phenoxyl radicals. The L-Nd complex is the easiest to oxidize, withE1=2 = 0.11, E21=2 = 0.21 and E31=2 = 0.34 V vs. Fc+/Fc, due to the larger size of the lanthanide ion. The ΔE1/2value (ΔE1/2 = E21=2 − E1=2) is correlated to the lanthanide radius, with values of 0.10 V for L-Nd and 0.22 V for L-Lu. The monoradical species were persistent in solution, allowing for their characterisation. All exhibit a distinct absorption band at around 445 nm due to the phenoxyl π–π* transitions. The EPR spec- trum of L-Lu+ consists of a single resonance at giso = 1.999, confirming the radical nature of the oxidized product. Most of the other complexes (L-Gd, L-Er, L-Yb) show a quenching of the LnIII-based resonances upon oxidation, indicative of magnetic interactions between the metal and the radical spins. The L-Ln (L = Nd, Er, Yb) complexes exhibit a metal-based luminescence upon excitation of the ligand. A significant quenching of the luminescence was observed upon radical formation: 92%, 83% and 79% respectively forL-Nd+, L-Er+ and L-Yb+.
More info
Date
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur Twitter
- Partager sur LinkedIn
- Share url